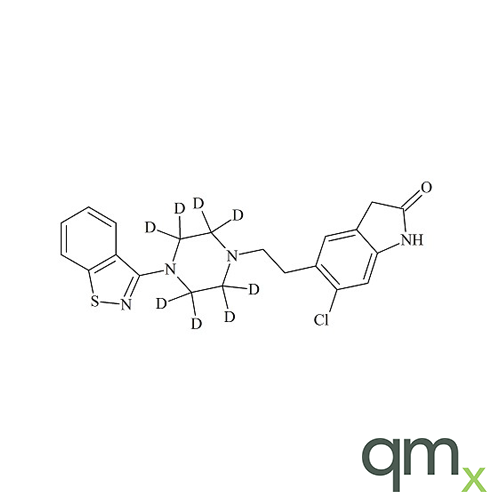
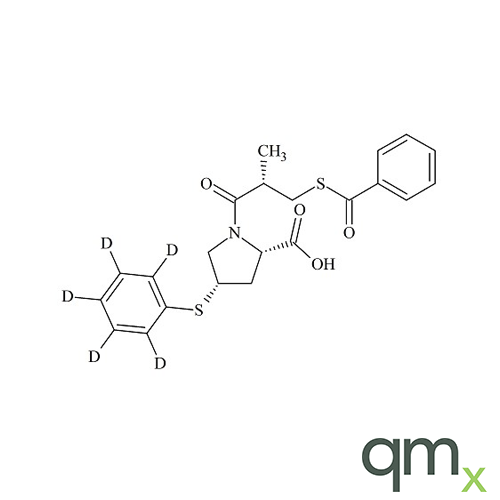
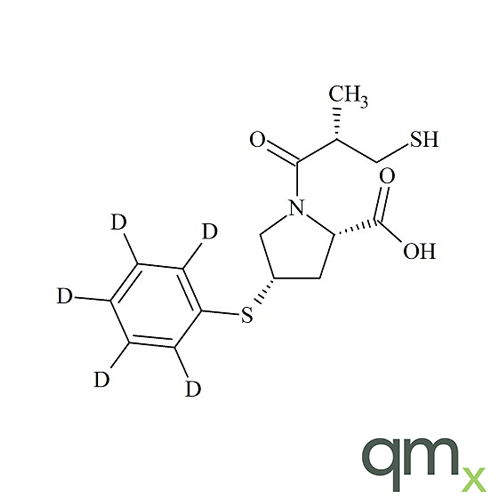
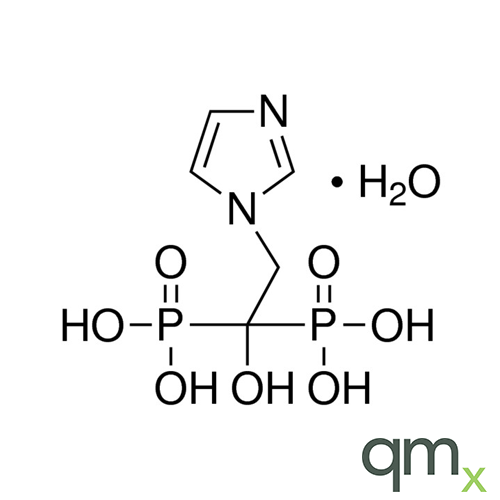
Pharmaceuticals
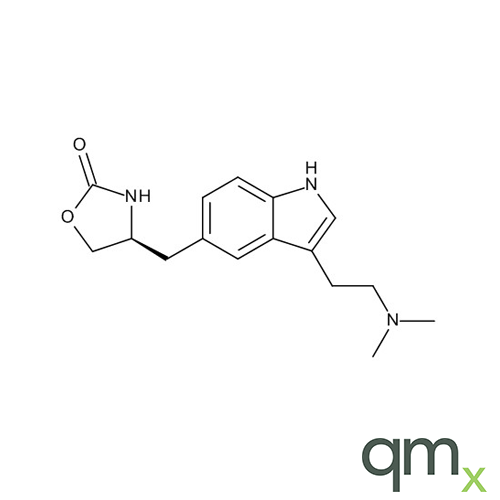
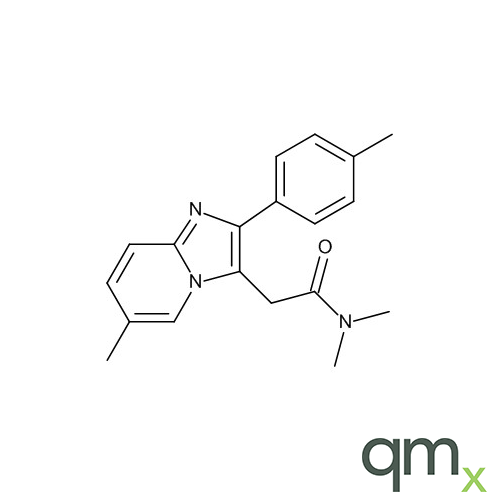
We offer an extensive range of reference standards for pharmaceutical and veterinary research, analysis and screening. The range includes reference standards of Active pharmaceutical ingredients, Metabolite and impurity products, Degradation products and process impurities, Inhibitor and receptor compounds, Genotoxic impurities and related substances for pharmacopeial updates.
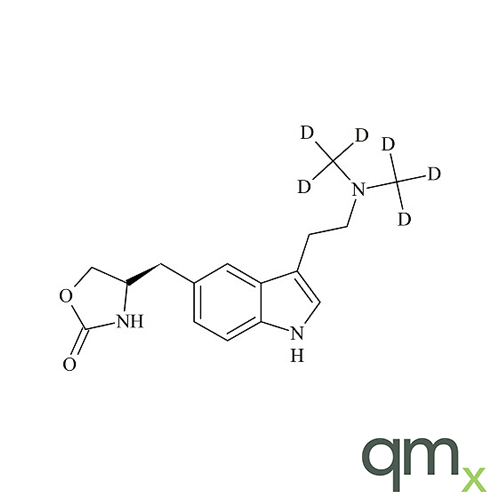
In addition, the range includes many isotopically labelled standards that offer the ideal solution to the problem of choosing a suitable internal standard for analysis.
If you are unable to locate the reference material or the format you need, please contact us with your requirement and we'll be delighted to check its availability.